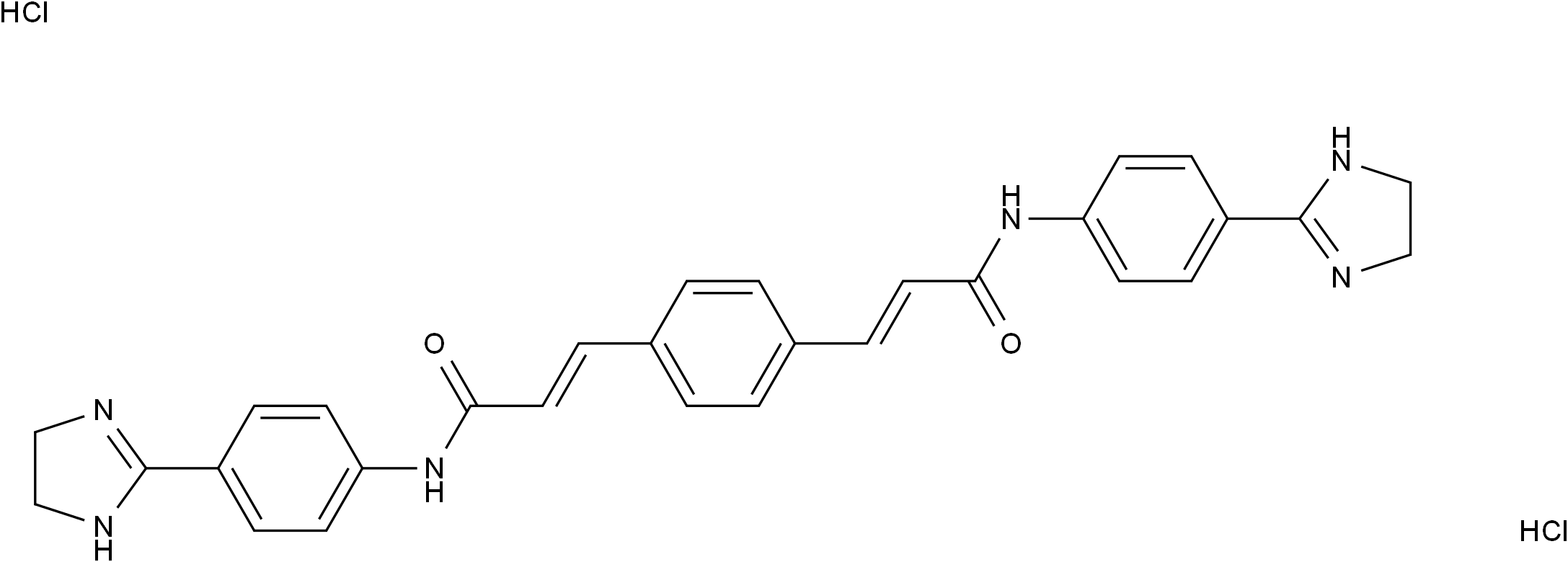
GW 4869
CAS No. 6823-69-4
GW 4869( —— )
Catalog No. M15607 CAS No. 6823-69-4
A cell-permeable, symmetrical dihydroimidazolo-amide compound that acts as a potent, specific, non-competitive inhibitor of N-SMase (neutral sphingomyelinase) [IC50?= ~ 1 μM, rat brain; Km?for sphingomyelin ~13 μM].
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 67 | In Stock |


|
| 5MG | 87 | In Stock |


|
| 10MG | 164 | In Stock |


|
| 25MG | 344 | In Stock |


|
| 50MG | 516 | In Stock |


|
| 100MG | 624 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameGW 4869
-
NoteResearch use only, not for human use.
-
Brief DescriptionA cell-permeable, symmetrical dihydroimidazolo-amide compound that acts as a potent, specific, non-competitive inhibitor of N-SMase (neutral sphingomyelinase) [IC50?= ~ 1 μM, rat brain; Km?for sphingomyelin ~13 μM].
-
DescriptionA cell-permeable, symmetrical dihydroimidazolo-amide compound that acts as a potent, specific, non-competitive inhibitor of N-SMase (neutral sphingomyelinase) [IC50?= ~ 1 μM, rat brain; Km?for sphingomyelin ~13 μM]. Does not inhibit human A-SMase (acid sphingomyelinase) even at 150 μM. Weakly inhibits the activities of bovine protein phosphatase 2A and mammalian lyso-PAF PLC, while no inhibition is observed for bacterial phosphatidylcholine-specific PLC. Reported to offer complete protection against TNF-α or diamine-induced cell death in MCF7 breast Y cells at 20 μM. Does not modify the intracellular glutathione levels or interfere with TNF-α or diamine-mediated signaling effects.
-
In VitroCell Viability Assay Cell Line:MCF7 human breast cancer cells.Concentration:10-20 μM.Incubation Time:30 min (then treated with TNF (3 nM) followed).Result:Significantly inhibited TNF-induced SM hydrolysis, whereas 20 μM of the compound protected completely from the loss of SM.Cell Viability Assay Cell Line:Fresh RAW264.7 macrophages.Concentration:10 or 20 μM.Incubation Time:2 hours (then treated with 1 μg/mL LPS incubation).Result:LPS-triggered exosome generation was remarkably attenuated in macrophages upon pre-treatment of macrophages with 10 μM GW4869, as evidenced by a 22% reduction in the activity of AChE. Such attenuation was further enhanced by treatment with the dose of 20 μM.
-
In VivoAnimal Model:10-12 weeks old Male wild-type C57BL/6 mice (Endotoxin-Challenged Mice).Dosage:2.5 μg/g.Administration:I.P. once (1 h later, followed by an i.p. injection of LPS (2.5 μg/g, 100 μL)).Result:Significantly decreased exosome levels by 37% in sera, compared to levels collected from control mice. At 12 h after LPS injection, the levels of circulating exosomes were increased significantly compared to PBS-controls, as evidenced by a 1.7-fold elevation in the AChE activity.Animal Model:10-12 weeks old Male wild-type C57BL/6 mice (CLP Polymicrobial Sepsis Model).Dosage:2.5 μg/g.Administration:I.P. once (before sham or CLP surgery).Result:Decreased exosome concentration by 33% compared to mice injected with PBS in sham-surgery controls. CLP-stimulated exosome release was significantly inhibited by pre-treatment of CLP mice compared to CLP mice pre-treated with PBS.
-
Synonyms——
-
PathwayMetabolic Enzyme/Protease
-
TargetPhospholipase
-
Recptorneutral sphingomyeliN/Ase
-
Research AreaCancer
-
Indication——
Chemical Information
-
CAS Number6823-69-4
-
Formula Weight577.5
-
Molecular FormulaC30H30Cl2N6O2
-
Purity>98% (HPLC)
-
SolubilitySoluble in DMSO
-
SMILESC1NC(=NC1)C2=CC=C(C=C2)NC(=O)/C=C/C3=CC=C(C=C3)/C=C/C(=O)NC4=CC=C(C=C4)C5=NCCN5.Cl.Cl
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Marchesini, N., et al. 2003. J. Biol. Chem. In press.
molnova catalog



related products
-
AACOCF3
AACOCF3 (Arachidonyl trifluoromethyl ketone) is a cell-permeable trifluoromethyl ketone analog of arachidonic acid and a potent, selective slow binding inhibitor of the 85-kDa cytosolic phospholipase A2 (cPLA2).
-
Sephin 1
Sephin1 is a selective inhibitor of holophosphatase. Sephin1 safely and selectively inhibited a regulatory subunit of protein phosphatase 1 in vivo.
-
ML-299
ML-299 is a dual PLD1/2 inhibitor (PLD1 and PLD2, IC50 of 6 nM, 20 nM, respectively).



 Cart
Cart
 sales@molnova.com
sales@molnova.com


